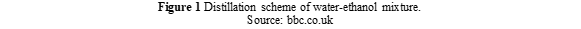- Your cart is empty
- Continue Shopping
Ethanol Purification
There is no doubt that ethanol is an alcohol with various purposes, from beverages to disinfectants. In organic synthesis, ethanol plays an important role in dissolving organic reagents and serves as a precursor to various useful products.
Commercial ethanol is available in numerous concentrations, from 70% to 95% by mass. The highest concentration, however, is not sufficient to be utilized in experimental works that require reagents with high purity.
Currently, fractional distillation is the common method to purify ethanol from its water mixture. Figure 1 illustrates the distillation scheme. This separation method is based on the boiling point difference between water and ethanol. Ethanol boils at a lower temperature than water (i.e., 78oC). When the mixture is heated to this temperature, ethanol boils and then passes through the condenser. As cold water from another source (such as a sink) flows into the condenser, ethanol will condense and collect at the collector. This method, however, limits ethanol purity to 95,6% by mass, due to the azeotropic mixture formed between water and ethanol.
Higher ethanol concentration, up to approximately 99%, has been achieved by alternative methods. For example, a research group from Bandung National Polytechnic used a mixture of Zeolite 3A and calcium oxide in a fractionating column. They managed to obtain ethanol with a concentration of up to 99,54% by volume (Ibrahim et al., 2022).
Reference:
Ibrahim, M. A., Musyaffa, M. H., Heriyanto, & Haryadi. (2022). Purification of Ethanol By Continuous Adsorption Method Using Zeolite 3a and Calcium Oxide. Jurnal Kimia Riset, 7(1), 9–19. https://doi.org/10.20473/jkr.v7i1.35682
BBC. (2024). Separating a liquid from a mixture – fractional distillation. Mixtures.
https://www.bbc.co.uk/bitesize/guides/zrqbbdm/revision/8