- Your cart is empty
- Continue Shopping
An Introduction to Gas Chromatography
Chromatography is an analytical separation method where the analytes partition between the mobile and stationary phases. Gas chromatography employs inert gases, such as nitrogen, helium, and argon gas, as the mobile phase. The analytes in the liquid or solution phase will be separated in the gas phase according to their boiling point and, to a lesser extent, their interaction with the stationary phase. The polar stationary phase will easily retain the polar solute and vice versa. Therefore, choosing the proper mobile and stationary phase is critical in achieving effective analyte separation. A schematic diagram of a gas chromatograph is exhibited in Figure 1.
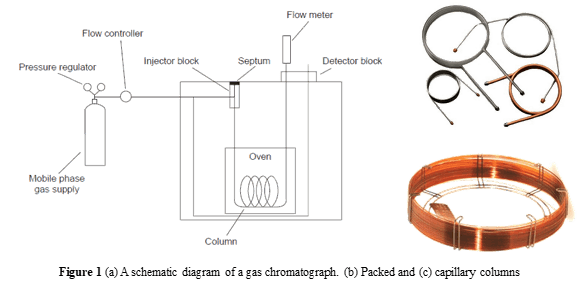
There are two widely used columns for gas chromatographs, i.e., packed and capillary columns. Packed columns are constructed of glass, stainless steel, copper, or aluminum and filled with particulate solid support whose diameter ranges from 37-44 μm to 250-354 μm. In gas-liquid chromatography, a liquid phase coated on a solid surface such as silica (SiO2) is employed as the stationary phase. To prevent solute adsorption on the exposed stationary phase, surface silanol (Si-OH) groups are deactivated through silanizing by dimethyldichlorosilane and then alcohol wash before coating with the stationary phase. In contrast to packed columns, capillary columns do not contain packing materials. Instead, they employ fused silica with a protective polymer.
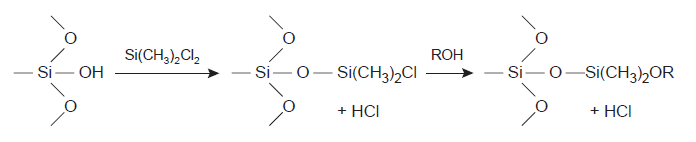
Gas chromatography can be employed for volatile samples, as the gaseous mobile phase must carry the analyte. The sample goes into the chromatogram through the injection block and undergoes vaporization in the oven.